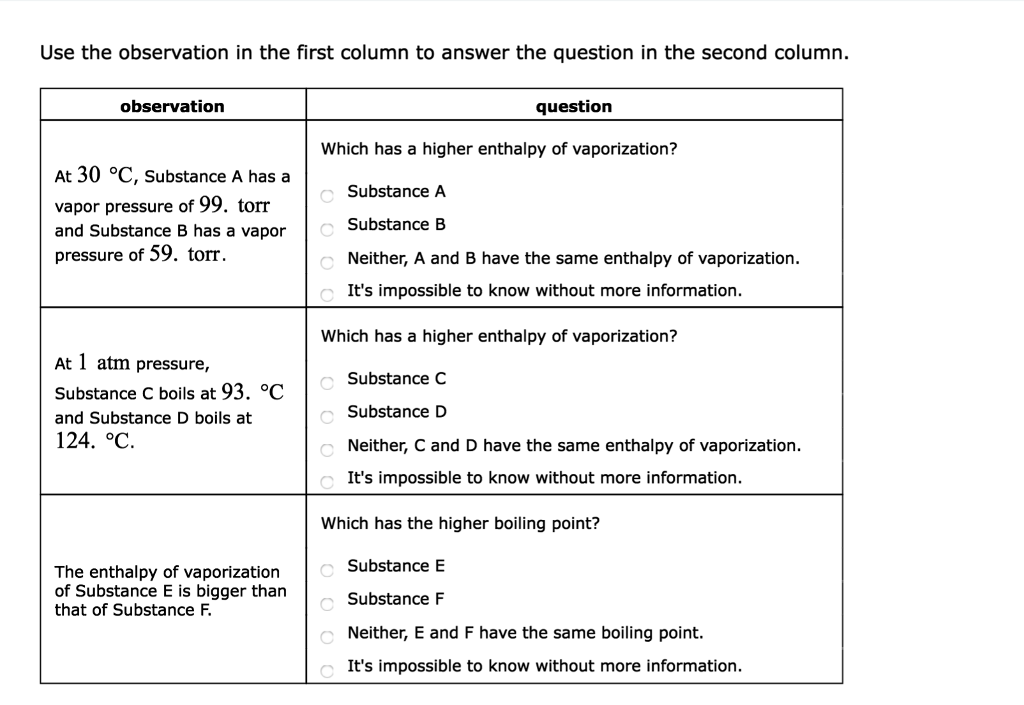
At Pressure Substance A Boils At And Substance Boils At
At 1 atm pressure, Substance C boils at -36 degree C and Substance D boils at -65 degree C Which has a higher enthalpy of vaporization?.
At pressure substance a boils at and substance boils at. Boils are painful, red bumps on the skin that are caused by bacteria. So what's going on?. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
It's impossible to know without more information. The boiling point of a liquid varies depending upon the surrounding environmental pressure. The majority phase is often called the what?.
A liquid which boils at a low temperature and evaporates rapidly at room temperature. The substance begins to freeze and boil at the same time. The vapor pressure of most liquids has a fairly predictable temperature-dependence, so from one boiling point measurement it is possible to give a good estimation of the boiling point at other pressures (or boiling pressure at other temperatures).
Heat required to vaporize or liquefy a substance at its boiling point. Temperature at which the vapor pressure of the solid and the vapor pressure of the liquid are equal. Substance C Substance D Neither, C and D have the same enthalpy of vaporization.
Learn how to get rid of a boil and what you can do at home and with your doctor to treat and prevent future boils. Of course, if we reduce the ambient pressure, the boiling point will be substantially reduced, and this is an example of the principle. In order for a substance to boil, the vapor pressure must equal what pressure?.
At one atmosphere of pressure, acrolein boils at 52.5 °C, and butane boils at –0.5 °C. The #"boiling point"# is defined as the temperature at which the vapour pressure of the liquid is equal to the ambient pressure, and bubbles of vapour form directly in the liquid.

Determine The Normal Boiling Point Of A Substance Whose Vapo Clutch Prep
Solved At 1 Atm Pressure Substance E Boils At 50 Degr Chegg Com

Tb Chapter11 bbbbbbbbbbbbbbbbbbbbbbbb
At Pressure Substance A Boils At And Substance Boils At のギャラリー
Boiling Chemistry Libretexts
Answered Jse The Phase Diagram Of Substance X Bartleby
Properties Of Liquids

Boiling Point Wikipedia
Q Tbn And9gct87xiufypzooanyid2gnr 0nrl8bmr3euhyw Usqp Cau
Solved Use The Observation In The First Column To Answer Chegg Com
A Vapor Pressure Lowering
What Is The Effect Of Pressure On A Boiling Point Quora
Define Melting Point And Boiling Point What Is The Difference Quora
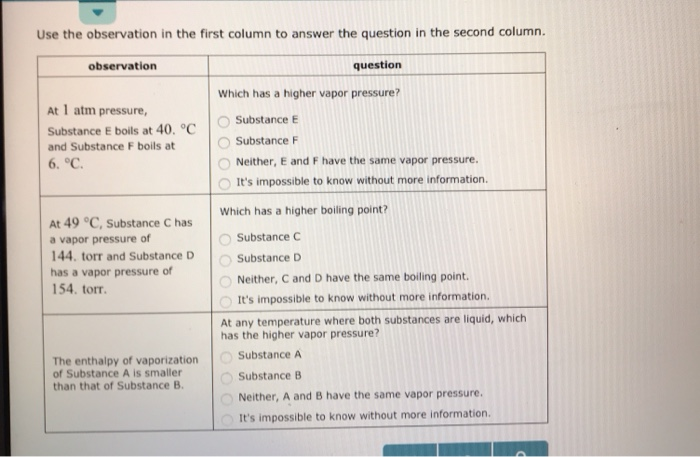
Solved Use The Observation In The First Column To Answer Chegg Com
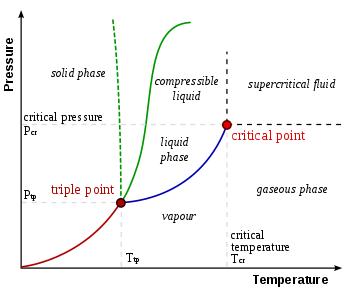
Triple Point Wikipedia

Aleks Calculating Vapor Pressure From Boiling Point And Enthalpy Of Vaporization Youtube
Q Tbn And9gcrdkbbenooxzb6w5cuyeljxk6jdbkkjf9dlt Oopmi3d6m6ih Usqp Cau

Answered If A Substance Has Stronger Bartleby

Determine The Normal Boiling Point Of A Substance Whose Vapo Clutch Prep

Solved Use The Observation In The First Column To Answer Chegg Com
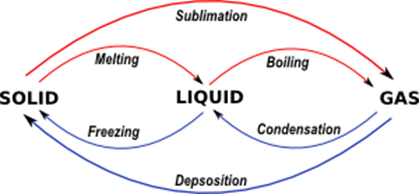
Change Of State Of A Matter Melting Fusing Evaporation Condensation
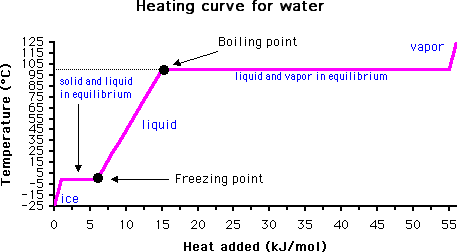
The Msds Hyperglossary Boiling Point

Distillation

The Boiling Point Of A Molecule Refers To Its Temperature At Which It Can Change From A Liquid State To That Of A Gas Or More Commonly Known As Vapour Copy
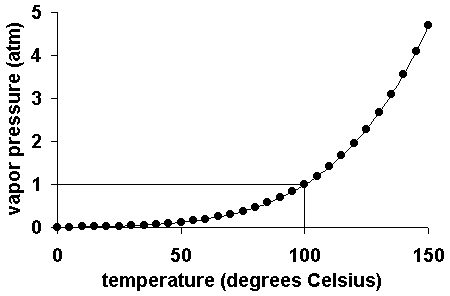
Vapor Pressure
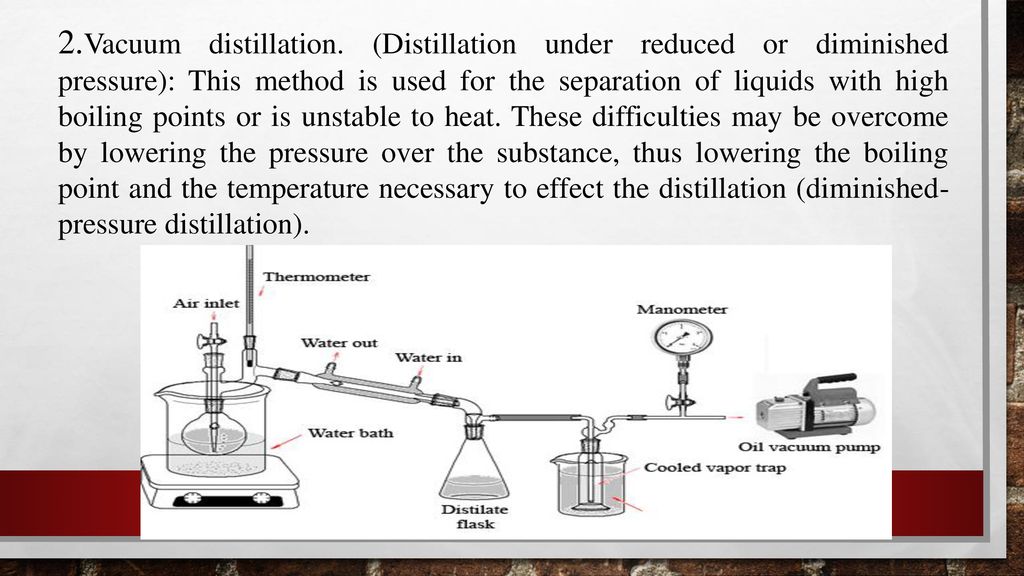
Experiment 4 Distillation Ppt Download

Separation By Distillation Scientific American
Vapor Pressure

Boiling Point Accessscience From Mcgraw Hill Education

Phase Diagrams

Melting Point Wikipedia
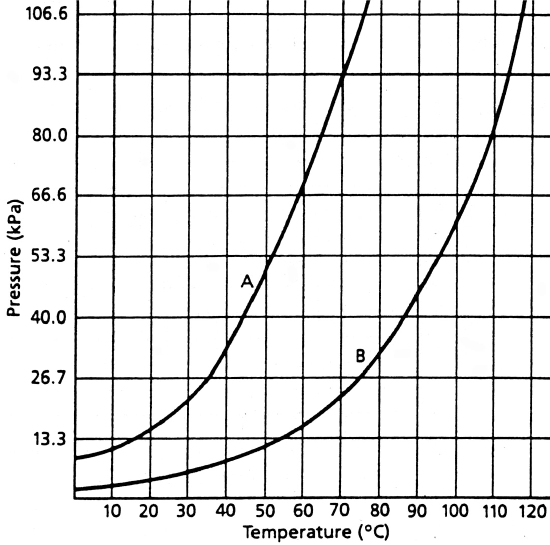
Worksheet 6 Solutions And Vapor Pressures Chemistry Libretexts
Vapor Pressure Wikipedia

Video Watch A Liquid Boil Freeze And Science Evidence Is Intelligence Facebook

Vapor Pressure Basic Introduction Normal Boiling Point Clausius Clapeyron Equation Chemistry Youtube
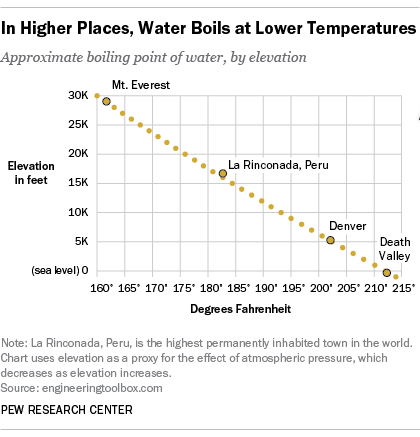
Does Water S Boiling Point Change With Altitude Americans Aren T Sure Pew Research Center
Solved Use The Observation In The First Column To Answer The Question In The Second Column Which Has A Higher Boiling Point At 98 C Substance Course Hero
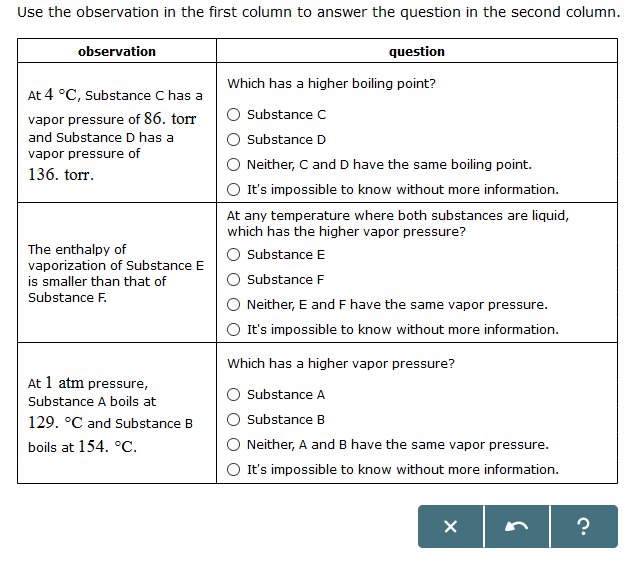
Solved Use The Observation In The First Column To Answer Chegg Com

Boiling Point Elevation Wikipedia

Boiling And Condensation Mini Physics Learn Physics

Tb Chapter11 bbbbbbbbbbbbbbbbbbbbbbbb

Determine The Vapor Pressure In Torr Of A Substance At 36c Whose Normal Boiling Course Hero

Boiling Chemistry Libretexts
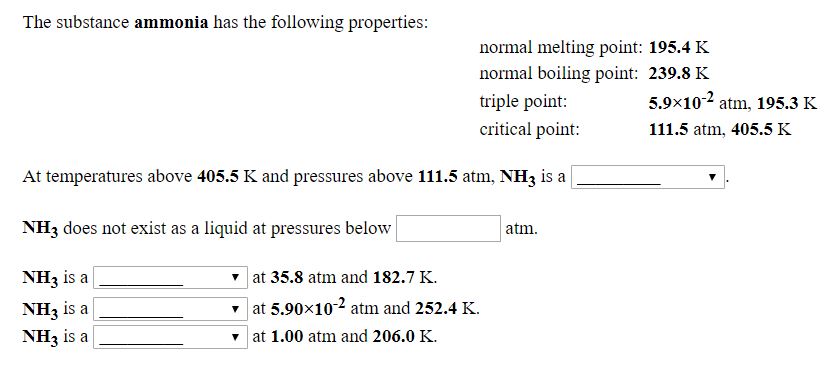
Answered The Substance Ammonia Has The Following Bartleby
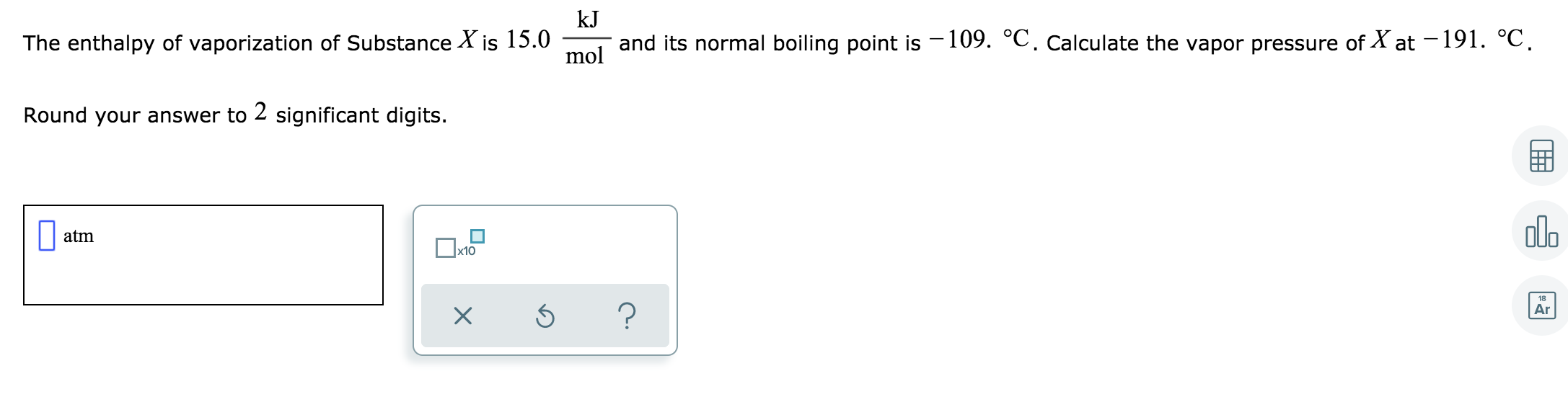
Answered Kj The Enthalpy Of Vaporization Of Bartleby

Phase Changes Boundless Chemistry

Differentiate States Of Matter Based On M P And B P A Substance Is Solid If Its

15 2d Understanding The Connection Between Vapor Pressure Boiling Point And Enthalpy Of Vaporizati Youtube
Chemfall09 Pbworks Com F Problem Set 4 Solutions Pdf

For The Choices Below What Is The Normal Clutch Prep
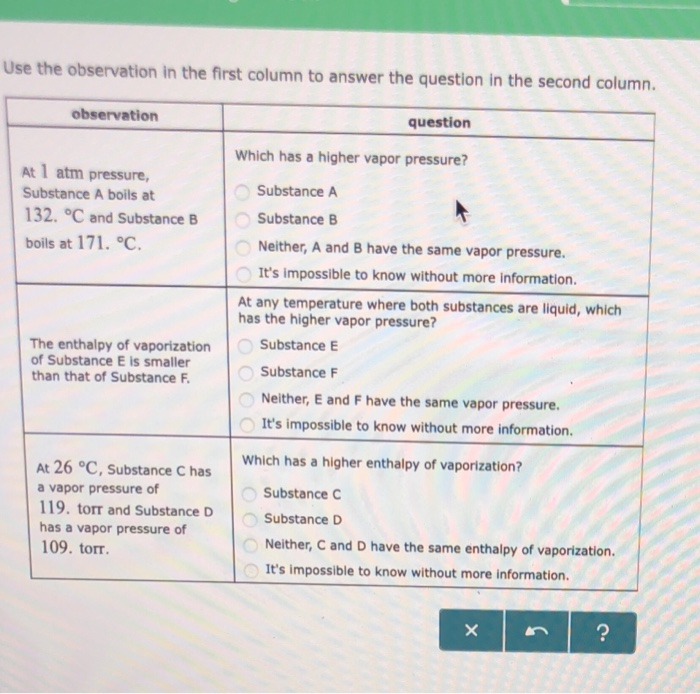
Solved Use The Observation In The First Column To Answer Chegg Com
Http Pubs Acs Org Doi Pdf 10 1021 Iea006

It S Scientifically Possible To Boil Water Until It Freezes Solid
Q Tbn And9gcsud5yxduujvoi51tzkhyocigbotoab35f24vsxzq54jba5yok8 Usqp Cau
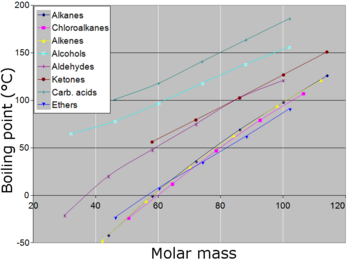
Boiling Point Wikipedia
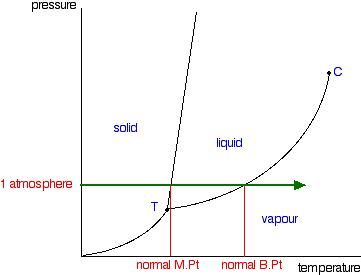
Phase Diagrams Of Pure Substances
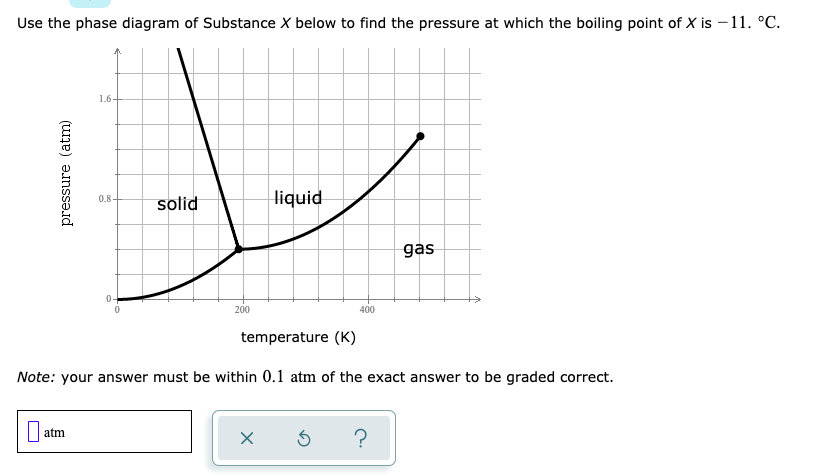
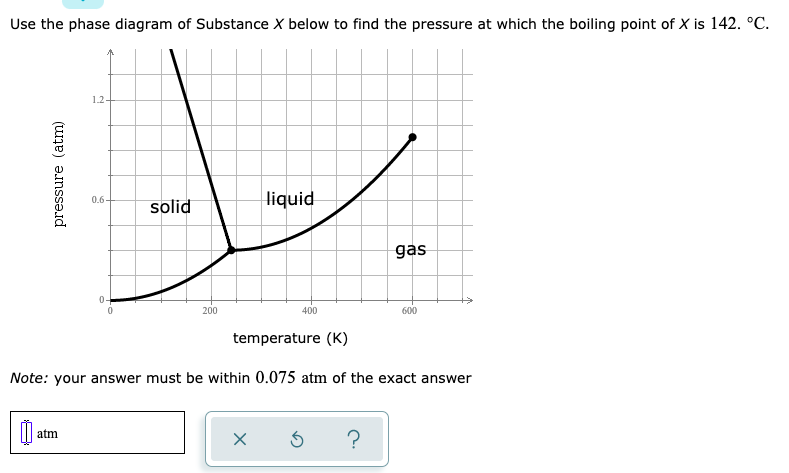
Answered Use The Phase Diagram Of Substance X Bartleby
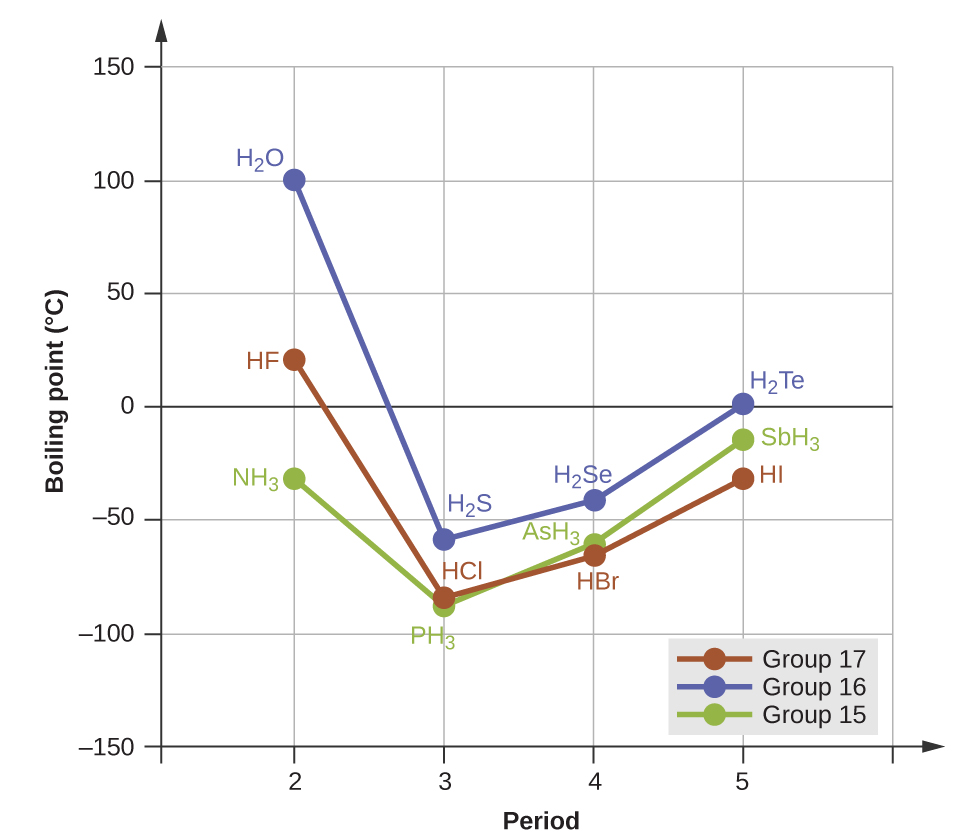
Melting And Boiling Point Comparisons M10q2 Uw Madison Chemistry 103 104 Resource Book
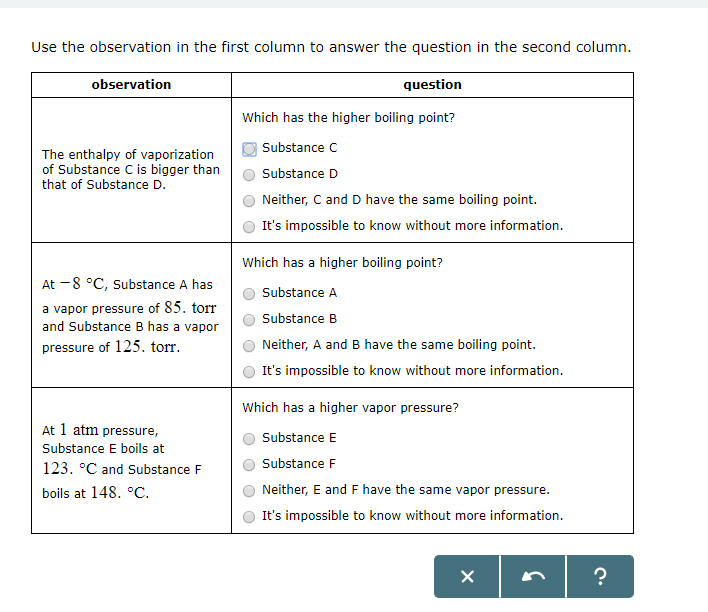
Solved Use The Observation In The First Column To Answer Chegg Com

Tb Chapter11 bbbbbbbbbbbbbbbbbbbbbbbb

Vapor Pressure Video States Of Matter Khan Academy
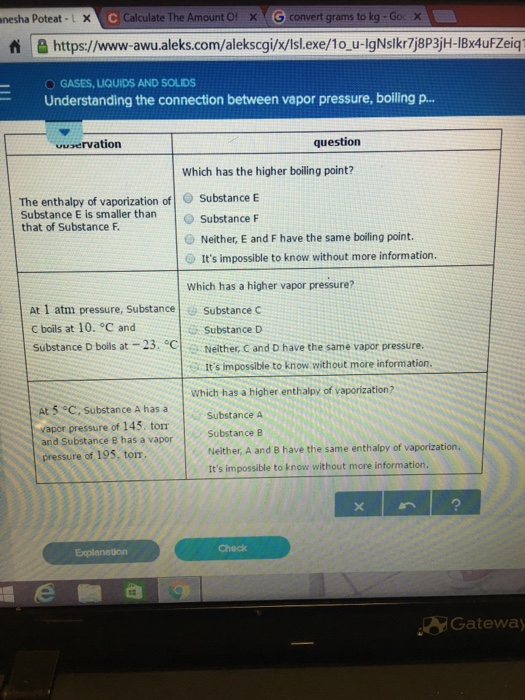
Solved The Enthalpy Of Vaporization Of Substance E Is Sma Chegg Com

What Temperature Does Water Boil At Boiling Point Elevation Compound Interest

Boiling Point Definition Examples Temperature Facts Britannica

A Substance Is Said To Be A If Its Melting Point Is Below Room Temperature And Boiling Point Is Above Room Temperature

Solved Use The Observation In The First Column To Answer Chegg Com
Characteristics Of The Solid Liquid And Gaseous States

Boiling Point From Pvt Diagram Example Youtube

Melting Point Freezing Point Boiling Point
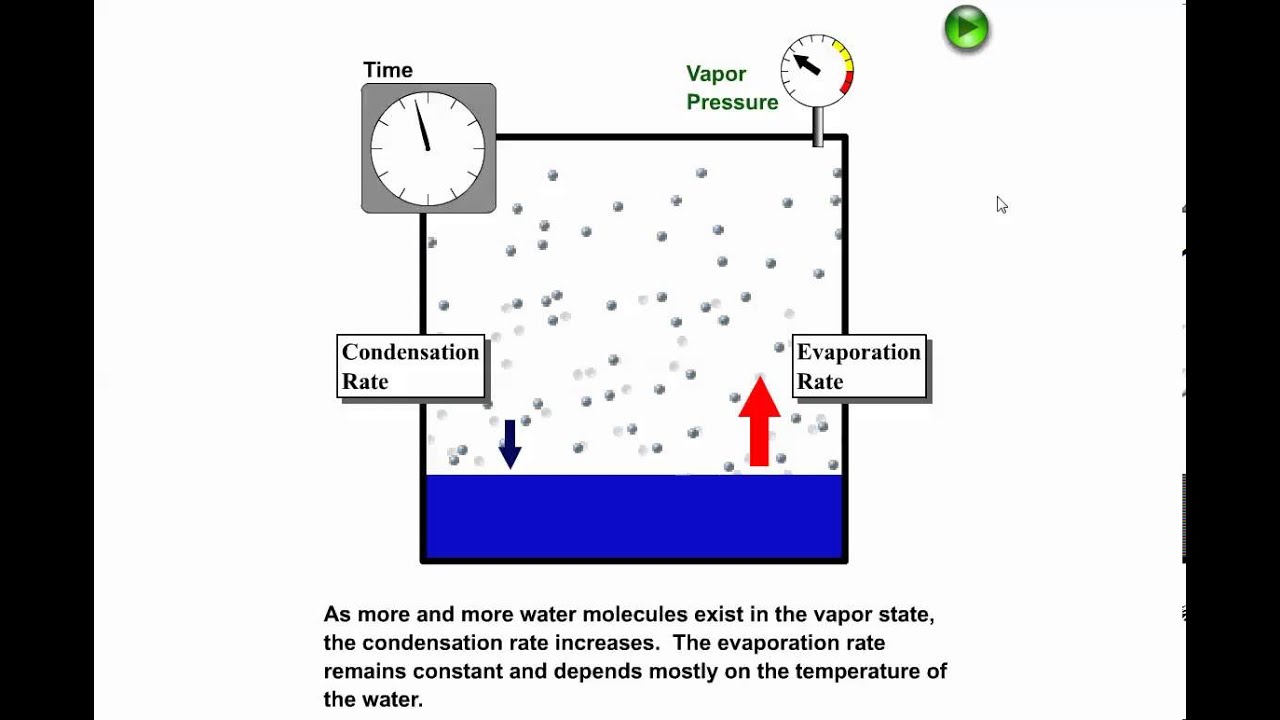
Phase Changes Boundless Chemistry
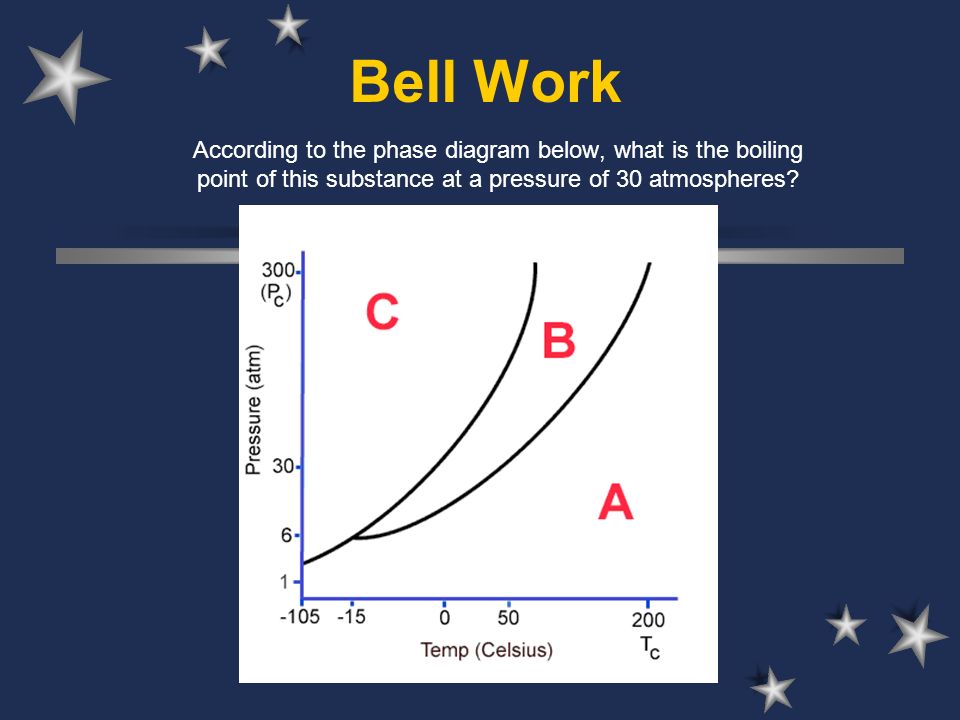
Bell Work According To The Phase Diagram Below What Is The Boiling Point Of This Substance At A Pressure Of 30 Atmospheres C Johannesson Ppt Download

The Vapor Pressure Of A Substance Describes How Readily Mole Clutch Prep
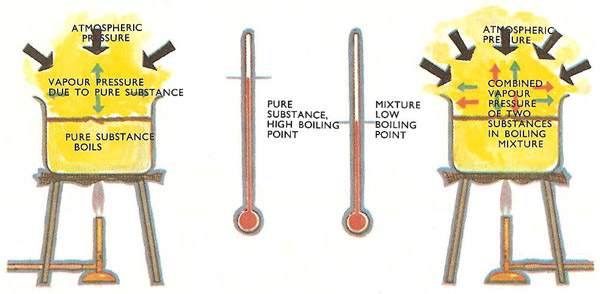
Steam Distillation
11 6 Properties Of Liquids Chemistry Libretexts
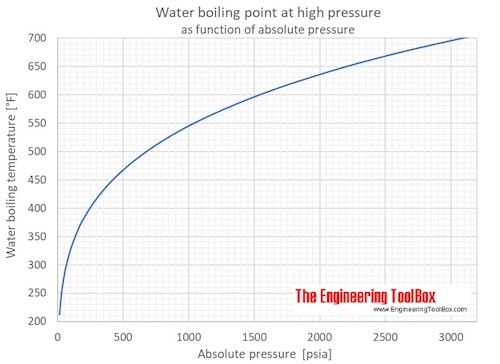
Water Boiling Points At Higher Pressure
Q Tbn And9gcqbjnwwgrewgb44etxgftigaqzpqvohpppjzogwltpwecbcjahb Usqp Cau
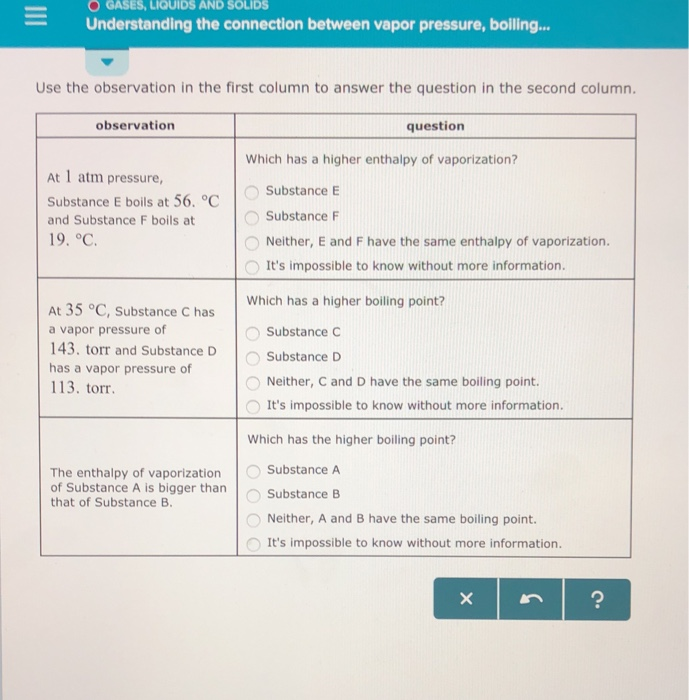
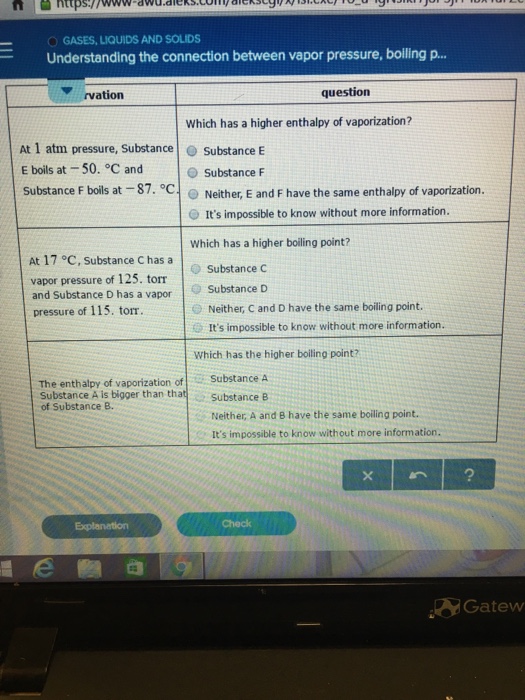
Solved Gases Liquids And Solids Understanding The Connec Chegg Com
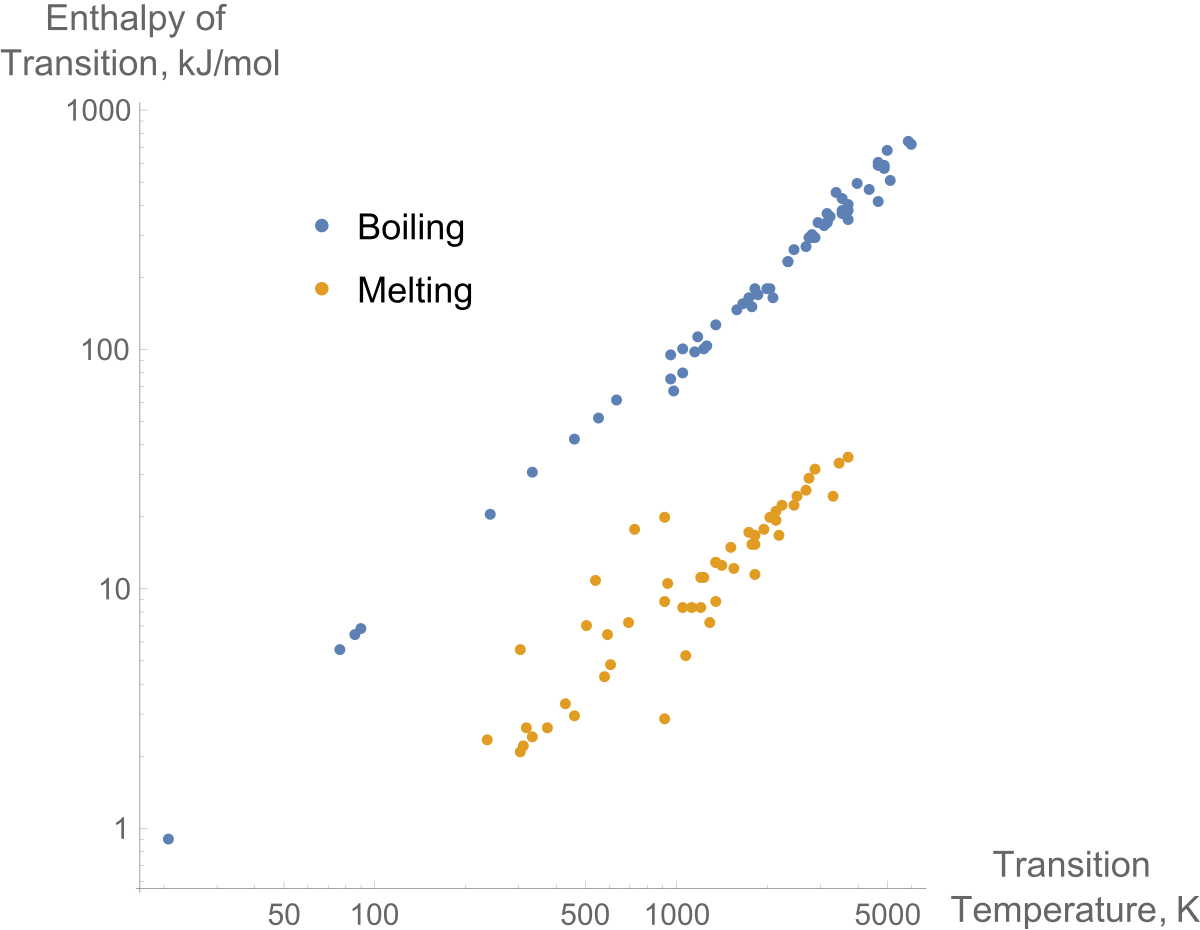
Enthalpy Of Fusion Wikipedia
2
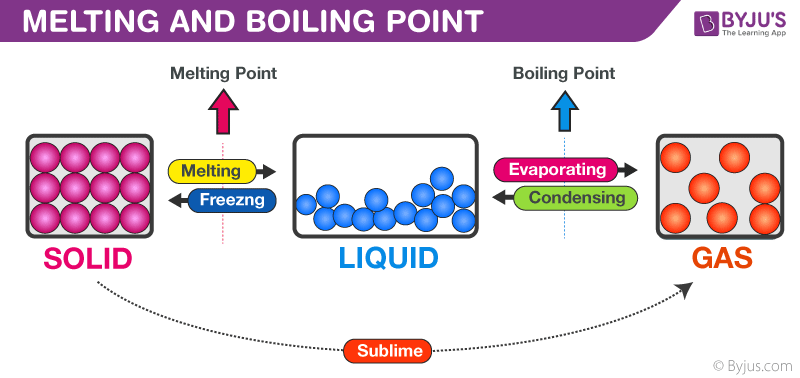
Melting Point Boiling Point Detailed Explanation With Videos
/glass-saucepan-on-a-gas-burner-with-boiling-water-dor961844-57fba8b03df78c690f79f7c6.jpg)
What Are The Bubbles In Boiling Water

11 5 Vaporization And Vapor Pressure Chemistry Libretexts
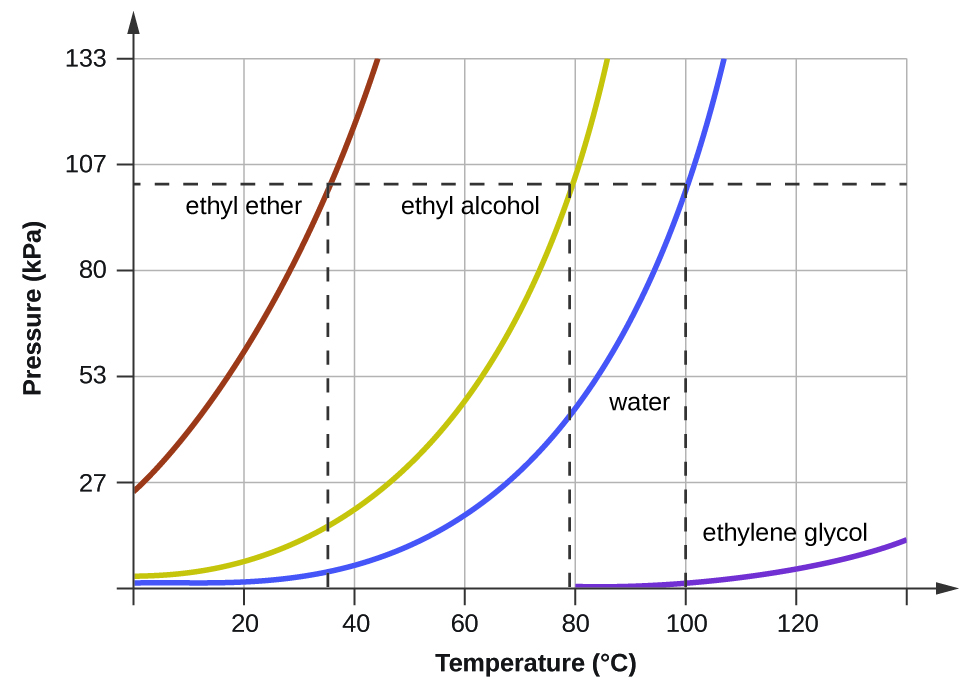
Vapor Pressure And Boiling Point Correlations M10q3 Uw Madison Chemistry 103 104 Resource Book
Q Tbn And9gctty78madvlpcjfr9 Hn4gxyqgpa El02cin0xy3jttw5cnyjnj Usqp Cau
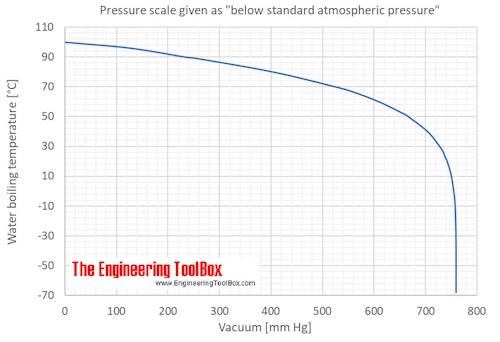
Water Boiling Points At Vacuum Pressure
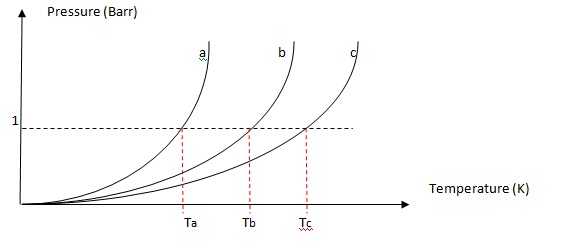
How To Find Boiling Point
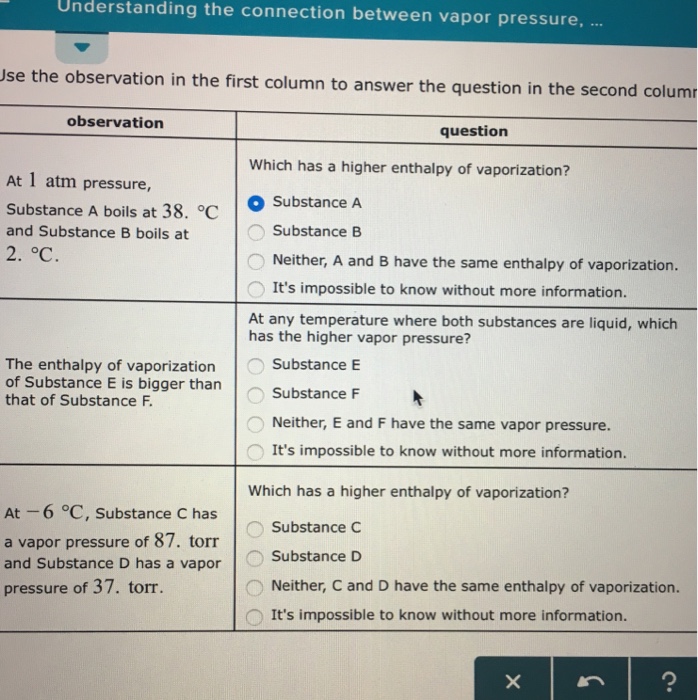
Understanding The Connection Between Vapor Pressur Chegg Com

If A Substance Has Weak Intermolecular For Clutch Prep

Attractions And Boiling

Boiling Point Of Chemical Compounds Mettler Toledo
Http Www Nhvweb Net Nhhs Science Bklingaman Files 12 08 Ch 10 Extra Review Key Pdf

Effect Of Pressure On The Boiling Point Of Water Youtube

Boiling And Condensation Mini Physics Learn Physics
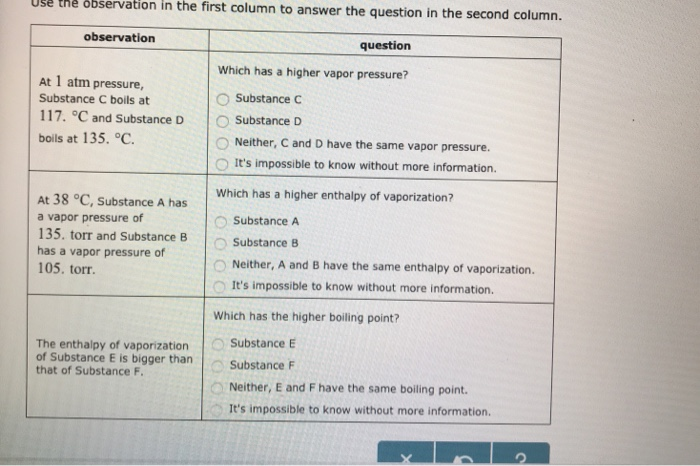
Solved Use The Observation In The First Column To Answer Chegg Com

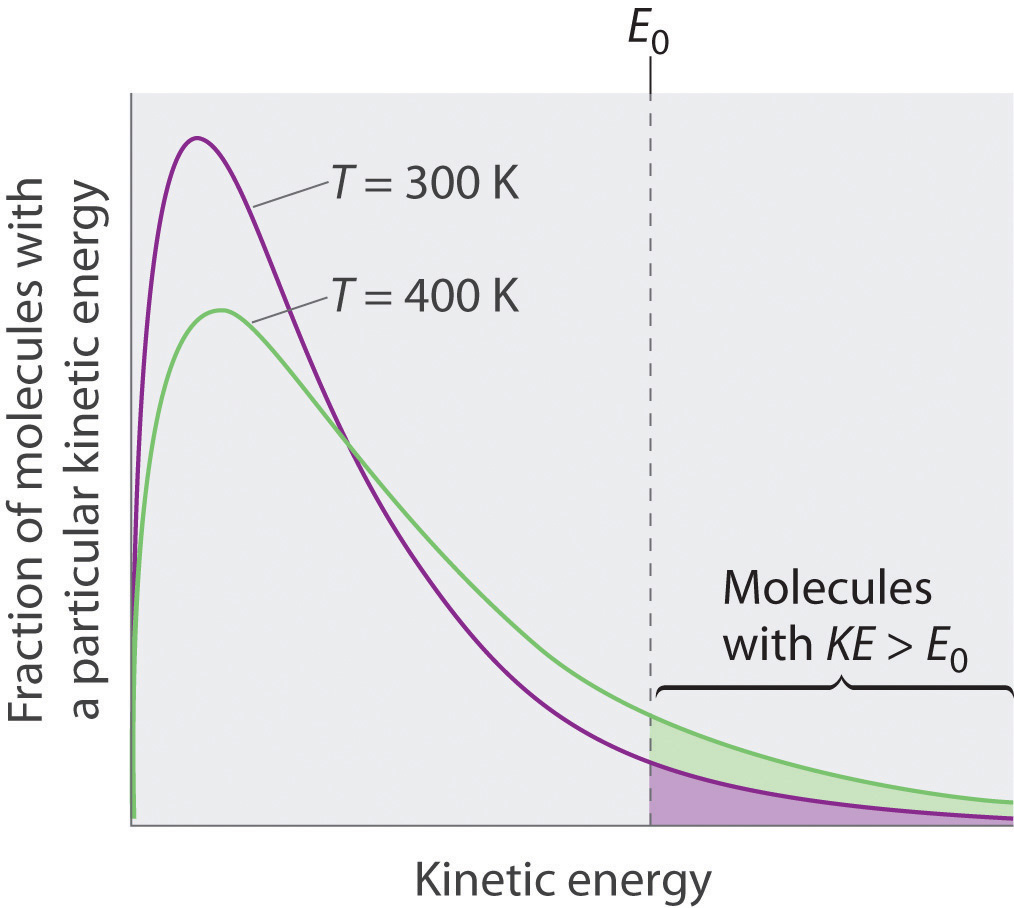
Lesson 2 Kinetic Molecular Theory

Solved Which Has A Higher Enthalpy Of Vaporization At 1 Chegg Com

The Normal Melting And Boiling Points Of A Substance Are 163 Degrees Celsius And 128 Degrees Celsius Respectively Its Triple Point Is At 125 K And 0 37 Atm Its Critical Point Is

Boiling Chemistry Libretexts

For The First Three Questions Use The Key Below

Vapor Pressure Curves Chemistry For Non Majors

Aleks Understanding The Connection Between Vapor Pressure Boiling Point Etc Youtube

Boiling Point Calculator
Solved Use The Observation In The First Column To Answer The Question In The Second Column Which Has A Higher Vapor Pressure At 1 Atm Pressure S Course Hero

Boiling Point Elevation And Freezing Point Depression Video Khan Academy
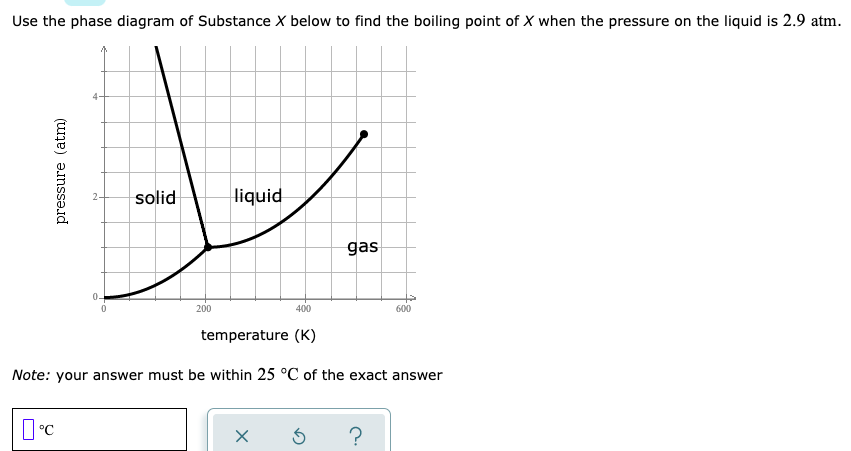
Answered Use The Phase Diagram Of Substance X Bartleby



